Check out Professor Dasgupta’s TED Talk, Driven to Care, which she presented at TedxEasthampton Women’s “Bold and Brilliant” series in Easthampton, Massachusetts.
Blog
Nanoparticals May Have Bigger Impact on the Environment Than Previously Thought
Research brief: Nanoparticles may have bigger impact on the environment than previously thought
First-of-its-kind study shows that non-antibacterial nanoparticles can cause resistance in bacteria.
In a first-of-its-kind study, researchers have shown that nanoparticles may have a bigger impact on the environment than previously thought. The research is published in Chemical Science, a peer-reviewed journal f the Royal Society of Chemistry.
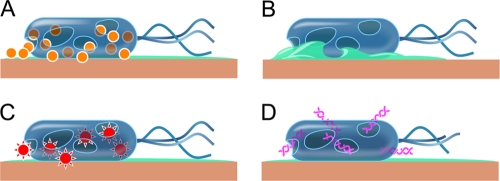
Researchers from the National Science Foundation Center for Sustainable Nanotechnology, led by scientists at the University of Minnesota, found that a common, non-disease-causing bacteria found in the environment, called Shewanella oneidensis MR-1, developed rapid resistance when repeatedly exposed to nanoparticles used in making lithium ion batteries, the rechargeable batteries used in portable electronics and electric vehicles. Resistance is when the bacteria can survive at higher and higher quantities of the materials, which means that the fundamental biochemistry and biology of the bacteria is changing.
“At many times throughout history, materials and chemicals like asbestos or DDT have not been tested thoroughly and have caused big problems in our environment,” said Erin Carlson, a University of Minnesota chemistry associate professor in the University’s College of Science and Engineering and the lead author of the study. “We don’t know that these results are that dire, but this study is a warning sign that we need to be careful with all of these new materials, and that they could dramatically change what’s happening in our environment.”
Carlson said the results of this study are unusual because typically when we talk about bacterial resistance it is because we’ve been treating the bacteria with antibiotics. The bacteria become resistant because we are trying to kill them, she said. In this case, the nanoparticles used in lithium ion batteries were never made to kill bacteria.
This is the first report of non-antibacterial nanoparticles causing resistance in bacteria.
In the past, many studies in the field exposed bacteria to a large dose of nanoparticles and observed if the bacteria died. This study was different because it looked at what happens over a more extended period of time to test how the bacteria might adapt over multiple generations when continually exposed to the nanoparticles. The bacteria were clearly able to take higher and higher doses of these materials over time without dying.
“Even though a nanoparticle may not be toxic to a microbe, it can still be dangerous,” said Stephanie Mitchell, a University of Minnesota chemistry graduate student and lead graduate student on this study.
Carlson warns that the results of this study go far beyond just bacteria.
“This research is very important to humans because bacteria are prevalent in our lakes and soil where there is a delicate balance of organisms. Other organisms feed on these microbes and there could be a major effect up the food chain or these resistant bacteria could have other effects we can’t even predict right now.”
Carlson said the researchers will continue follow-up studies to determine the effects of other human-made nanomaterials on other organisms in the environment and the long-term effects.
“Research that both advances technology and sustains our environment is a priority for the Division of Chemistry,” said Michelle Bushey, program director for the Chemical Centers for Innovation Program at the National Science Foundation. “This work reveals unexplored and long-term impacts that some nanoparticles have on the living organisms around us. This discovery at the chemistry-biology interface is a first step toward developing new sustainable materials and practices, as well as providing the groundwork for possible remediation approaches.”
###
In addition to Carlson and Mitchell, other lead researchers on the study include University of Minnesota Chemistry Professor Christy Haynes, Augsburg University Chemistry Associate Professor Z. Vivian Feng, and University of Wisconsin-Madison Chemistry Professor Robert Hamers, the director of the Center for Sustainable Nanotechnology. Others on the research team include University of Minnesota researchers Natalie Hudson-Smith, Meghan Cahill, and Benjamin Reynolds; Augsburg University researchers Seth Frand and Rodrigo Tapia Hernandez; and University of Wisconsin-Madison researchers Curtis Green and Chenyu Wang.
This research was funded by the National Science Foundation through the Center for Sustainable Nanotechnology, an NSF Center for Chemical Innovation.
To read the full research paper, visit the Chemical Science website.

IMAGE: ERIN CARLSON, A UNIVERSITY OF MINNESOTA CHEMISTRY ASSOCIATE PROFESSOR, LED THE TEAM FROM THE NSF CENTER FOR SUSTAINABLE TECHNOLOGY THAT THAT SHOWED FOR THE FIRST TIME THAT NON-ANTIBACTERIAL NANOPARTICLES CAN… view more
CREDIT: PATRICK O’LEARY, UNIVERSITY OF MINNESOTA
Emma Gustafson at the Santa Fe Opera
Emma Gustafson, a current Augsburg senior in the Theater Department, has spent the last two summers in the internship of her dreams: building and styling wigs for the Santa Fe Opera in New Mexico. A competitive apprenticeship for young theatre designers and technicians, this opportunity was a once in a lifetime experience for Emma. I asked her to share her thoughts on this incredible step into the real world of theatre.
Photos pictured here and more can be found on Emma’s Instagram.

Could you describe a typical day at the Santa Fe Opera?
“Days are long at the Santa Fe Opera– 6 days a week, sometimes 12 hour days, but every minute is worth it! You get to work full time doing what you love and your opportunity for growth is immeasurable! It’s like bootcamp for your craft, but in the beautiful high-desert and with an on-campus pool.
“As a Wigs Apprentice, I spent my days building, coloring, cutting, and styling wigs, facial hair, and other hair pieces. The nights were spent running shows–each night is a different opera and there are always 5 in rotation for the entire summer! While running a show, I would be responsible for putting wigs and makeup on singers and assisting with backstage changes or quick changes.”
What was your biggest takeaway from the experience?
“My biggest takeaway was simply the craft itself, the Santa Fe Opera gave me the opportunity to work with some of the best-of-the-best in such a niche industry.”
What are some ways that the Augsburg Theater department prepared you for this internship?
“Having amazing mentors and teachers such as Sarah Bahr, who helped me find and land this job, and Michael Burden who encouraged me to work professionally and me taught about the stagecraft of theater, I was able to go into SFO (nervous, yes, but) confident enough with background knowledge to engage with a professional and prestigious company.”
More about Emma:
As a Theater Design and Technical major and Graphic Design minor in the Class of 2020, Emma plans to begin working in the theater industry in the area of Hair, Makeup and Wigs as a designer, run-crew, and wig builder after graduation. She is also the student director in Augsburg’s 2019-2020 season; she will be directing “Quake” by Melanie Marnich–to be performed in January/February 2020!

This post was written and contributed by Jenny Moeller, Administrative Assistant to the Art, Communication Arts, and Theater Departments. Thanks, Jenny and Emma!
The Augsburg University Greenhouse: Final Friday Flower Hour
This blog entry was contributed by Dr. Leon van Eck, Assistant Professor of Biology and Hagfors Greenhouse Curator.
The Augsburg University Greenhouse is located on the 4th floor of the Hagfors Center. It is a little over 500 square feet in size, but is packed with sophisticated technology. The climate is computer-controlled through an Argus Control System, which allows for automatic control of the lighting, the heating and cooling systems, and the shade curtain. The only thing we don’t control automatically is the watering, and that’s because of the diverse needs of our growing plant collection. Over the past year as Assistant Professor and Hagfors Greenhouse Curator, I have collected together over 250 different plant species to support research and teaching activities in the Biology Department. These plants span the gamut, from delicate mosses and ferns, tropical vines and orchids, carnivorous plants, to food plants not typically seen in Minnesota, like pineapple and papaya. Students enrolled in BIO361 Plant Biology get the opportunity to get up close with these plants and flex their botany skills. A highlight this semester has been the flowering of the voodoo lily (Amorphophallus konjac), a slightly smaller (and even smellier!) relative of the infamous titan arum.

Our plant collection focuses on crop wild relatives, those species of plants that are closely related to familiar plants like tomato and barley, but that harbor useful traits such as resistance to drought and disease not found in the commercial cultivars. These plants are of particular interest as we think ahead to sustainably feeding a growing population in the face of climate change. Another focus of our collection is the biodiversity of the Horn of Africa. Since Augsburg University is in the heart of a vibrant Northeast African community, we are well-positioned to showcase the amazing, important, and threatened plants from that part of the world. This includes some striking succulent species, as well as plants like frankincense and myrrh, specimens of which are currently thriving on the rooftop of the Hagfors Center.

The greenhouse has recently obtained a vertical gardening system, which we installed and planted up over the summer, to have a lush green wall and even more opportunities to get students excited about plants. This wall features many cliff-dwelling species, including an impressive collection of rainforest cacti.
The Plant Growth Facilities at Augsburg not only house the Biology Department’s permanent plant collections, but are also a place of active science. Students conduct original URGO-sponsored research on trichome density and the microbiomes of Minnesota-native wild strawberries, study the disease resistance genomics of barley, and conduct experiments on plant community interactions for BIO481 Ecology. There are many opportunities for students to gain hands-on experience in the plant sciences, right here in the Hagfors Center!

My current favorite plant in our collection? A spiny succulent called Edithcolea grandis, which produces large flowers patterned like a Persian carpet. This exotic relative of the humble milkweed is named after the intrepid female botanist Edith Cole, who collected it in the mountains of northern Somalia in 1895. It is these kinds of evocative stories that can really bring the world of botany alive in people’s imaginations, and help me in my mission to show people how fascinating and important the plant kingdom can be.
If you’d like to know what we’ve got “growing on”, and see the plant collection in person, the doors of the Hagfors Greenhouse are open to the Augsburg community on the last Friday of every month for Final Friday Flower Hour. Greenhouse staff will be available to showcase our plant collections and research projects from noon to 2PM. I hope you’ll discover a new favorite species.
– Dr. Leon van Eck,
Assistant Professor, Biology Department
Hagfors Greenhouse Curator
3D Printing Food with Noah Aleshire
Augsburg Physics Major and Summer Researcher, Noah Aleshire, has been hard at work conducting investigations into the science of 3D printing food. After seeing a number of intriguing Twitter posts from Physics Professor, Ben Stottrup, I asked Noah to provide some information about himself and his work for the new Arts and Sciences blog.
The photos included all came from Professor Stottrup’s posts.

What made you want to research 3D printing food?
“I think this project stemmed from (Professor) Stottrup’s interest in food science and my interests in mechanical engineering. I expressed interest in using 3D printing to help me explore high-powered rocketry for my aerospace club, which sparked the idea of blending our interests and attempting to 3D print food. This would allow me to explore 3D printing while also helping advance Augsburg’s exploration of food science. Not to mention, broadening the “making” community by introducing the capability of printing various types of food pastes (frosting, chocolate, mashed potatoes, etc.). Having a printer that can print food will undoubtedly draw more students towards “making” and to food science. We also are thinking of collaborating with Campus Kitchen to host a few events that utilize/showcase our ability to print food by potentially printing designs on cakes or just designs that could be cooled and then placed onto a cake.
“The printers we got came as a DIY kit, so I spent over a week building and learning the intricate components. This allowed me to modify our printer, as needed, and design different methods of paste extrusion (I show my two major designs in the poster). Now having built a 3D printer (I also own one at home), I am currently building a CNC router for the shop downstairs.
How was your research funded?
“My work was funded by the generous donations of Dean and Amy Sundquist through Augsburg’s Department of Undergraduate Research and Graduate Opportunities, while also funded by NASA’s Space Grant.”
More About Noah:
- He is President of Augsburg’s Society of Physics Students (Spring 2018 – Spring 2020) and President of Augsburg’s Unofficial Aerospace Club (a student-run subset of the Society of Physics Students).
- He has a Level 1 High-Powered Rocketry certification through Tripoli, MN Rocketry Association (he can build and launch rockets of a certain class that generally go under 5,000 ft) and plans to go for Level 2 this school year (generally under 10,0000 ft).
- This will be his senior year at Augsburg. He will graduate in 2020 with a Bachelor of Science in Physics and a Minor in Mathematics.
- He is applying for PhD programs in Aerospace Engineering this fall; PhD in Mechanical Engineering is his second consideration.


Augsburg Faculty Team Chosen for Competitive Active Learning in Science Seminar
(Minneapolis) – An Augsburg University faculty team was selected as one of 10 from a competitive, national pool of applicants to participate in a new program designed to prepare faculty members to adopt active learning methods proven to be successful in teaching science.
Associate Professor of Biology Jennifer Bankers-Fulbright was the lead applicant and, along with Biology Lecturer Teresa Krause and Physics Department Chair Benjamin Stottrup, learned to implement new methods based on the research findings of Stanford University professor of physics and Nobel laureate Carl E. Wieman. These methods are designed to improve teaching effectiveness and student learning in biology, chemistry, and physics courses.
The summer 2019 seminar was offered by the Council of Independent Colleges (CIC) and supported by a $300,000 grant from the W. M. Keck Foundation.
“The ability to think like a scientist is critical for all students, not just those who will major in STEM or plan to pursue an advanced degree,” said Richard Ekman, the CIC president. “Systematic change is needed to create the science-literate population needed to understand research-based science policy, which affects all aspects of today’s society.”
Although small colleges have long been recognized for the high percentages of their science majors who complete undergraduate degrees, earn advanced degrees, and enter STEM careers, this seminar marks the first systematic attempt to promote this powerful pedagogy among faculty members at smaller independent colleges and universities. Wieman provided the inspiration for and has been the guiding force in developing the seminars, recommending the facilitators, providing the syllabus, and shaping the process.
Despite numerous studies that have demonstrated improved effectiveness if instruction were changed from traditional lectures to more effective, active learning methods—in the sciences as in other fields—research indicates that the lecture is still the default method for many faculty members.
Each institution supported a team of four faculty members from no more than two disciplines (biology, chemistry, or physics), including at least one department or division chair or dean. The team received intensive training to prepare them to implement and assess research-based active learning methods in introductory courses in their departments when they return to campus.
The first seminar took place July 15–19, 2019, at Holy Names University in Oakland, California. After the seminar, college faculty members will participate in webinars, as well as conference calls and a site visit for each institution.
Contact: Gita Sitaramiah, director of PR and internal communications, 612-330-1476.
About Augsburg: Augsburg University offers more than 50 undergraduate majors and 10 graduate degrees to 3,400 students of diverse backgrounds at its campus in the vibrant center of the Twin Cities and nearby Rochester, Minnesota, location. Augsburg educates students to be informed citizens, thoughtful stewards, critical thinkers, and responsible leaders. An Augsburg education is defined by excellence in the liberal arts and professional studies, guided by the faith and values of the Lutheran church, and shaped by its urban and global settings. Learn more at Augsburg.edu.
The Council of Independent Colleges (CIC) is an association of 770 nonprofit independent colleges and universities, state-based councils of independent colleges, and other higher education affiliates, that works to support college and university leadership, advance institutional excellence, and enhance public understanding of independent higher education’s contributions to society. CIC is the major national organization that focuses on services to leaders of independent colleges and universities and state-based councils. CIC offers conferences, seminars, publications, and other programs and services that help institutions improve educational quality, administrative and financial performance, student outcomes, and institutional visibility. It conducts the largest annual conferences of college and university presidents and of chief academic officers. Founded in 1956, CIC is headquartered at One Dupont Circle in Washington, DC. For more information, visit www.cic.edu.
The Copper Touch
The intention of this blog is to highlight the accomplishments and experiences of Augsburg’s faculty, staff, and students within the divisions of Fine Arts/Humanities and Natural/Social Sciences. Our first post was written by Augsburg University Chemistry student, Seth Frand, and shared by Associate Professor of Chemistry, Vivian Feng. It was originally posted on June 12, 2019 to the Sustainable Nano blog, hosted by the Center for Sustainable Nanotechnology (CSN). Check the Arts and Sciences page regularly for more posts.
When you hear “copper,” is the first thing that comes to your mind “medicine”? Probably not. You likely think of pennies (which are actually composed of mostly zinc here in the US), or maybe you think of wiring and conductors for electronics. But, believe it or not, copper has had a long history of medicinal use. Records from ancient societies like the Egyptians and Greeks tell of copper being used to treat a variety of ailments; from blurry vision and cataracts to digestive disorders and parasites, but most commonly as topical treatment of wounds.(1) The ancients also used copper in drinking vessels as a means of sterilizing drinking water.(2) In fact, inorganic remedies like this persisted into the modern age, falling out of favor with the advent of antibiotics in the early 1930s.

But this begs the question, what’s copper got going on here? How can a common metal have such a big impact that its use lasted thousands of years? As it turns out, copper is capable of a phenomenon known as “contact killing,”(3) which is exactly what it sounds like. When microbes come in contact with it, they stop living. This occurs because copper is an essential micronutrient, and bacterial cells will take in copper ions under the assumption that they are getting their nutrients. Unfortunately for the little microbes, they eventually take in a lethal dose of ions. Too much copper can have a myriad of effects that interfere with regular cell functions, like maintaining osmotic pressure or preventing oxidative stress, and eventually cause the cell to stop functioning all together. To give you an idea of how little copper is really needed for nutrition, an adult human (with about 37 trillion cells!) needs at most 10 mg a day.
Trouble for the bacteria means benefits for humans. Due to copper’s property of contact killing, the EPA has recognized copper as the first solid antimicrobial material, with over 300 alloys listed as such in 2008. Some medical facilities make use of this by using copper alloys on touch surfaces.(5) These are places like handles on doors and faucets or hand rails along staircases, areas where multiple people frequently touch. Thanks to contact killing, any germs left behind by someone turning a handle or holding a hand rail will likely die. The purpose of this is to reduce the amount of bacteria transferred from person to person by preventing build-up of microbes on commonly handled surfaces. At the same time, these surfaces pose no dangers to people as copper has not been shown to absorb through the skin.(6) Comparing standard stainless-steel surfaces to copper during repeated contamination over the course of 24 hours shows the benefit of using copper instead of steel for touch surfaces.

Aside from finding a place in hospitals, antimicrobial copper has been employed in areas where people commonly come into contact with one another and risk transferring illnesses. For example, Hartsfield-Jackson Atlanta International Airport has retrofitted the drinking fountains with copper surfaces. Athletic training centers, such as the Gilmour Academy Ice Arena, uses custom made copper grips on weight lifting equipment.(8) Even fast food chain Chick-fil-A has installed copper door handles on restrooms in a few locations.(5)
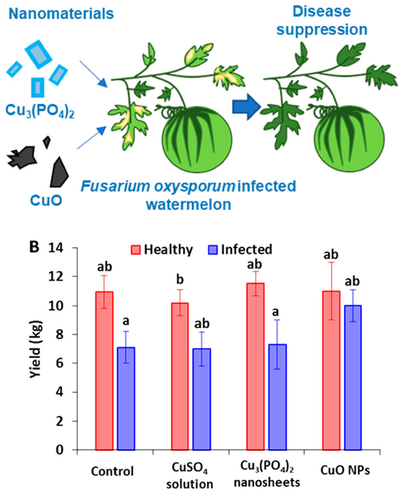
Beyond being used to fight bacterial disease in humans, copper can be used in agriculture to combat disease in plants as well. It is a common micronutrient added to fertilizers, and many applications to combat crop disease take advantage of copper at the nanoscale. Research has shown that copper can kill fungus as well as bacteria, and there are many types of easily obtainable copper-based fungal treatments for house plants, gardens, and farms.(9)
Conventional copper anti-fungal treatments have downsides, however. A lot of fungicides use copper salts as their source for copper metal. In chemistry, a salt is an ionic compound, meaning one portion carries a positive charge and the other portion carries a negative charge. These charges attract one another and form a crystal – ordinary table salt (NaCl) is a perfect example. When it comes to using copper salts to kill fungus on plants, there can be an inadvertent introduction of nitrates, sulfates, or other components of the salt compounds to the environment. Using copper nanoparticles instead of copper salts is one way to avoid these extraneous salt components. The nanoparticles can be applied directly by spraying a solution on the leaves of the plant, rather than into the soil around it. This helps to both prevent and reverse infection in some very popular crops, like coffee, cocoa, tea, and bananas.(10) Very good news if you drink as much coffee as I do!
So it turns out copper is useful for more than just minting pennies. Aside from being a nutrient essential to bacterial and plant growth, it’s able to inhibit bacterial and fungal infections in some important situations. Its amazing ability to induce contact killing makes it a valuable supplement in the fight to prevent disease. From hospitals to gyms and even restaurants, copper fittings and fixtures can help combat bacterial growth and the spread of potential illnesses. And in gardens and farms it can help plants grow well and fight infections.
Despite these amazing applications, it’s important to remember that copper is just a supplement, not a substitute, to maintaining cleanliness for disease prevention. We all still need to clean up common areas and wash our hands regularly! It’s a bit of a wonder how society let this amazing metal fall to the wayside in our excitement with the discovery of antibiotics. Fortunately, this metal is starting to make a comeback, and soon maybe everyone will think about medicine as well as coins when copper is mentioned.
References
- The Copper Development Association. The Sumerians and Chaldeans. (n.d.)
- Sowder, A. Ancient Greek Bronze Vessels. In Heilbrunn Timeline of Art History. New York: The Metropolitan Museum of Art, 2000–. April 2008.
- Zeiger M, Solioz M, Edongué H, Arzt E, Schneider AS. Surface structure influences contact killing of bacteria by copper. Microbiologyopen. 2014;3(3):327–332. doi:10.1002/mbo3.170
- Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Applied Environmental Microbiology. 2010;77(5):1541–1547. doi:10.1128/AEM.02766-10
- Sun, L. The bacteria-fighting super element that’s making a comeback in hospitals: copper. The Washington Post, Sept 20, 2015.
- Agency for Toxic Substances and Disease Registry. Public Health Statement: Copper. CAS# 7440-50-8. Sept 2004.
- Usman, M. S., et al. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. International Journal of Nanomedicine. 2013, 8, 4467. Doi: 10.2147/IJN.S50837
- CleanLink. Case Study: Ice Arena Reduces Risk Of MRSA, Other Bacteria With Antimicrobial Fixtures. Nov 14, 2004.
- Stone, A. & Baker, B. Organic Management of Late Blight of Potato and Tomato with Copper Products. eOrganic, March 18, 2010.
- Gianessi, L. & Williams, A. Without Fungicides, World Banana Exports Would Collapse. CropLife Foundation, August 2011.
- Borgatta, J., Ma, C., Hudson-Smith, N., Elmer, W., Plaza Pérez, C.D., De La Torre-Roche, R., Zuverza-Mena, N., Haynes, C.L., White, J.C. and Hamers, R.J., 2018. Copper Based Nanomaterials Suppress Root Fungal Disease in Watermelon (Citrullus lanatus): Role of Particle Morphology, Composition and Dissolution Behavior. ACS Sustainable Chemistry & Engineering, 6(11), 14847-14856. Doi: 10.1021/acssuschemeng.8b03379

